Currently, cancer patients who experience recurrence of disease face a challenging prognosis and limited new treatment options – especially in glioblastoma (brain cancer), colorectal, pancreatic, ovarian, and small cell lung cancers.
High toxicity levels in available marketed treatments can lead to their discontinuation, due to tolerability and/or quality of life issues.
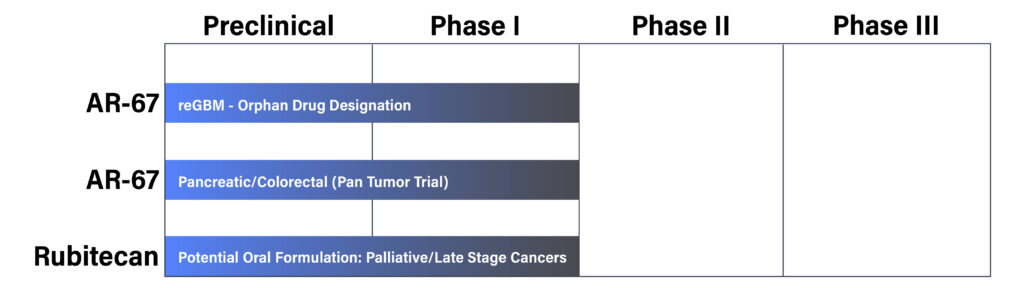
Vivacitas’ lead program AR-67, is a lipophilic small molecule compound with a proprietary synthesis method developed to address especially hard-to-treat tumors and improve tolerability and patient comfort.
Using breakthrough technology to evolve current treatments and improve patient quality of life.
AR-67 is a new generation of treatment, building on its current strengths and advancing it further.
Our patented synthesis method has the potential to transform the current oncology treatment space.
Our expert team brings a scientific pedigree of vision and tenacity to push innovation forward.
Preclinical, Phase I, and Phase II clinical data for AR-67 already shows promise for treatment of tough-to-treat cancers such as recurrent glioblastoma.
The patient is at the heart of everything we do and fuels our ambitions to accelerate innovation for scientific discovery.

1. Novel silatecan displays high lipophilicity, improved blood stability and potent
anticancer activity. Bom D, et al J Med Chem 2000; 43:3970-3980
2. Silatecan DB-67 is a novel DNA Topo-1 targeted radiation sensitizer: Chen AY. Mol
Cancer Ther 2005; 4(2): 317-24.
3. Phase I study publication: Arnold SM, et al. Clin Cancer Res. 2010;6:673-680
4. Phase II study publication (abstract): Kumthekar P, et al. SNO 2019. Poster ACTR-40,
published in Neuro-Oncology(https://academic.oup.com/neuro-oncology)
5. Ubiquitin-dependent Destruction of Topoisomerase I Is
Stimulated by the Antitumor Drug Camptothecin*, Desai et al. The Journal of Biological Chemistry, Vol. 272, No. 39, Issue of September 26, pp. 24159–24164, 1997. https://www.jbc.org
6. Metabolic Pathways of the Camptothecin Analog AR-67, Horn et al. Drug Metabolism and Disposition, Vol. 39, No. 4, 37390/3672838, 2011. https://dmd.aspetjournals.org